An FDA warning about droopy eyelids, blurred vision and fake Botox in nine states
“Harmful reactions” from women in nine states led to alerts from the FDA and CDC this week about fake Botox, unlicensed medical facilities and unlicensed people giving the shots.
Here’s what’s known.
Where did the ‘harmful reactions’ happen? Who got harmed?
The agencies say the “adverse event” reports came from 19 women in Florida, Kentucky, Illinois, New York, New Jersey, Colorado, Nebraska, Tennessee and Washington. The youngest was 25, the CDC said, and the oldest 59.
Nine women were hospitalized and four were given botulism antitoxin to prevent the botulinum toxin (Botox) from spreading to other parts of the body.
“Eighteen people reported receiving [Botox] injections for cosmetic purposes,” the CDC said. “All people reported receiving these injections from unlicensed or untrained individuals or in non-healthcare settings, including homes and spas.”
What are the symptoms of a poorly done Botox shot?
Botulism commonly causes labored breathing, a hard time swallowing, weakened musculture, droopy eyelids and blurred vision or “seeing double.”
In addition to those symptoms, the CDC and FDA said, the 19 women dealt with incontinence, dry mouth, slurred speech, fatigue, difficulty raising the hea; and general weakness.
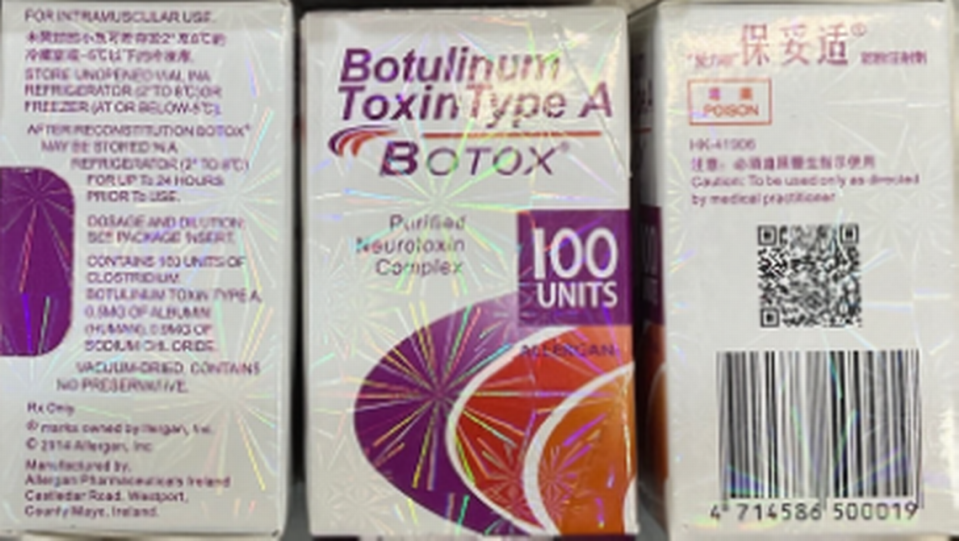
What do counterfeit Botox boxes and bottles look like?
The FDA said signs of the bogus Botox include:
▪ Lot No. C3709C3 on the bottle and box.

▪ Declaration of 150-unit doses on the bottle and/or box. The FDA says neither AbbVie nor Allergan makes 150-unit doses.

▪ The active ingredient for Botox should be “OnabotulinumtoxinA.” On the boxes for fake Botox, it’s “Botulinum Toxin Type A.” instead of “OnabotulinumtoxinA.”
▪ The box “contains language that is not English.”
What you should do?
Get Botox shots from a licensed professional in a medical office or setting and make sure it’s really Botox.

